| Thermodynamics | |||||||||

| |||||||||
Classical thermodynamics describes the thermal interaction of systems individually in thermodynamic equilibrium. Non-equilibrium thermodynamics may be considered separately as an extension to classical theory using the tools of statistical thermodynamics which describes all systems as ensembles of microscopic states.
The four principles, or laws, of thermodynamics are:[1][2][3][4][5][6]
- The zeroth law of thermodynamics provides a basic definition of empirical temperature based on the principle of thermal equilibrium.
- The first law of thermodynamics mandates conservation of energy and states in particular that the flow of heat is a form of energy transfer.
- The second law of thermodynamics states that the entropy of an isolated macroscopic system never decreases, or, equivalently, that perpetual motion machines are impossible.
- The third law of thermodynamics concerns the entropy of a perfect crystal at absolute zero temperature, and implies that it is impossible to cool a system to exactly absolute zero.
The laws of thermodynamics have become some of the most important fundamental laws in physics and other sciences.
Contents |
Zeroth law
The zeroth law of thermodynamics may be stated as follows:When two systems, each internally in thermodynamic equilibrium at a different temperature, are brought in diathermic contact with each other they exchange heat to establish a thermal equilibrium between each other.If two thermodynamic systems are each in thermal equilibrium with a third system, then they are in thermal equilibrium with each other.
The zeroth law implies that thermal equilibrium, viewed as a binary relation, is a transitive relation. Thermal equilibrium is furthermore an equivalence relation between any number of system. The law is also a statement about measurability. To this effect the law establishes an empirical parameter, the temperature, as a property of a system so that systems in equilibrium with each other have the same temperature. The notion of transitivity permits a system, for example a gas thermometer, to be used as a device to measure the temperature of another system.
Although the concept of thermodynamic equilibrium is fundamental to thermodynamics, the need to state it explicitly as a law was not widely perceived until Fowler and Planck stated it in the 1930s, long after the first, second, and third law were already widely understood and recognized. Hence it was numbered the zeroth law. The importance of the law as a foundation to the earlier laws is that it defines temperature in a non-circular logistics without reference to entropy, its conjugate variable.
First law
The first law of thermodynamics may be stated as in several ways:Energy can be neither created nor destroyed. It can only change forms.
In any process in an isolated system, the total energy remains the same.
The first law of thermodynamics states that energy cannot be created or destroyed; rather, the amount of energy lost in a steady state process cannot be greater than the amount of energy gained. This is the statement of conservation of energy for a thermodynamic system. It refers to the two ways that a closed system transfers energy to and from its surroundings – by the processes of heat and mechanical work. The rate of gain or loss in the stored energy of a system is determined by the rates of these two processes. In open systems, the flow of matter is another energy transfer mechanism, and extra terms must be included in the expression of the first law.For a thermodynamic cycle the net heat supplied to the system equals the net work done by the system.
The first law clarifies the nature of energy. It is a stored quantity which is independent of any particular process path, meaning it is independent of the system history. If a system undergoes a thermodynamic cycle, whether it becomes warmer, cooler, larger, or smaller, then it will have the same amount of energy each time it returns to a particular state. Energy is a state function and infinitesimal changes in the energy are exact differentials.
All laws of thermodynamics but the first are statistical and simply describe the tendencies of macroscopic systems. They are only strictly valid in the thermodynamic limit when a system has many states. For microscopic systems with few particles the assumptions of thermodynamics become meaningless.
The first law may be expressed by several forms of the fundamental thermodynamic relation:
- Increase in internal energy of a system = heat supplied to the system + work done on the system
This is also often stated as a definition of the amount of heat of a process:
- Heat supplied to a system = increase in internal energy of the system + work done by the system
Second law
The second law of thermodynamics may be summarized as follows:The second law states that spontaneous natural processes increase entropy overall, or in another formulation that heat can spontaneously flow only from a higher-temperature region to a lower-temperature region, but not the other way around. Entropy is increased also by processes of mixing without transfer of heat.When two isolated systems in separate but nearby regions of space, each in thermodynamic equilibrium in itself, but not in equilibrium with each other at first, are at some time allowed to interact, breaking the isolation that separates the two systems, and they exchange matter or energy, they will eventually reach a mutual thermodynamic equilibrium. The sum of the entropies of the initial, isolated systems is less than or equal to the entropy of the final exchanging systems. In the process of reaching a new thermodynamic equilibrium, entropy has increased, or at least has not decreased.
The second law defines entropy, which may be described as a measure of ignorance or uncertainty of the microscopic details of the motion and configuration of the system given only predictable reproducibility of bulk or macroscopic behavior. For example, one has less knowledge about the separate fragments of a broken cup than about an intact cup, because when the fragments are separated, one does not know exactly whether they will fit together again, or whether perhaps there is a missing shard. Crystals, the most regularly structured form of matter, with considerable predictability of microscopic configuration, as well as predictability of bulk behavior, have small values of entropy; and gases, which behave predictably in bulk even when their microscopic motions are unknown, have high entropy.
The entropy of an isolated macroscopic system never decreases. However, a microscopic system may exhibit fluctuations of entropy opposite to that stated by the second law.
S≥0
Third law
The third law of thermodynamics is usually stated as follows:As temperature approaches absolute zero, the entropy of a system approaches a minimum.




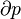

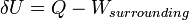

No comments:
Post a Comment